Abstract
Introduction
Venous thromboembolism (VTE) is a common complication of cancer. Patients with primary brain tumors and brain metastases are at particularly high risk, with about 20-30% suffering a VTE event. The administration of enoxaparin appears to be safe in patients with brain metastases but confers an increased risk of intracranial hemorrhage (ICH) in patients with primary brain tumors. Direct-acting oral anticoagulants (DOACs) have demonstrated efficacy in the treatment of cancer-associated thrombosis with an increased risk of hemorrhage compared to low molecular weight heparin (LMWH). There are limited data on the safety of DOACs in patients with brain tumors. As the risk of ICH associated with parenteral anticoagulants differs for primary versus secondary brain tumors, we analyzed ICH outcomes for patients with VTE receiving enoxaparin or a DOAC.
Methods
A retrospective cohort study was performed using a hospital-based online medical record database (CQ2) linking ICD-9 and ICD-10 codes with prescription medication records. Cases were identified based on coding for primary brain tumors or brain metastases and prescription of either a DOAC or enoxaparin. A blinded review of radiographic imaging was performed, and intracranial hemorrhages were categorized as either trace, measurable, and major. Measurable intracranial hemorrhages were those defined as greater than 1 mL in volume and major intracranial hemorrhages were defined as greater than 10 mL in volume, symptomatic (defined as focal deficit, headache, nausea, or a change in cognitive function), or required surgical intervention. Gray's test was used to compare the cumulative incidence of ICH between the groups, with death as a competing risk.
Results
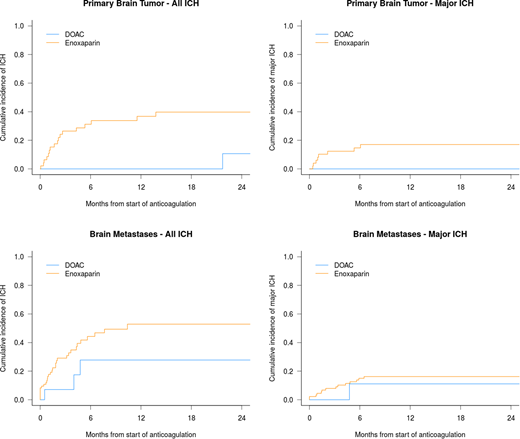
A total of 170 patients with primary brain tumors and brain metastases were included in the study. In the primary brain tumor cohort (N=65), 18 patients received a DOAC while 47 received enoxaparin. The cumulative incidence of any ICH at 12 months was 0% in patients receiving a DOAC compared to 36.8% (95% confidence interval 22.3-51.3%) in those receiving enoxaparin (P=0.012). There were no major ICH events in the DOAC group and 8 and in the LMWH group (12-month cumulative incidence of 0% versus 18.2%, 95% CI 8.4-31.0, P=0.062). In the brain metastases cohort (N=105), 21 patients received a DOAC while 84 received enoxaparin. The DOAC and enoxaparin groups were well-matched for tumor diagnosis (non-small lung cancer 52% and 51%, respectively) including those tumor types with a high incidence of ICH (i.e. melanoma 5% and 7% and renal cell carcinoma 14% and 11%, respectively). In patients with brain metastases, DOACs did not increase the risk of any ICH relative to enoxaparin (12-month cumulative incidence 27.8% versus 52.9%, P=0.15) nor major ICH (12-month cumulative incidence 11.1% vs 17.8%, P=0.38).
Conclusions
DOACs can be safely administered to patients with brain tumors. In patients with primary brain tumors (i.e. glioma), DOACs appear to be safer than LMWH and should be considered for this indication.
Zwicker:Incyte: Research Funding; Parexel: Consultancy; Quercegen: Research Funding; Daiichi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal